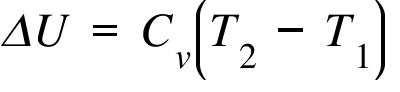functional analysis - Why does $\int_{\Omega}\Delta u \frac{\partial u}{\partial t}dx=-\frac{1}{2}\frac{d }{d t}|u|^2_{H^1_0}$ hold? - Mathematics Stack Exchange
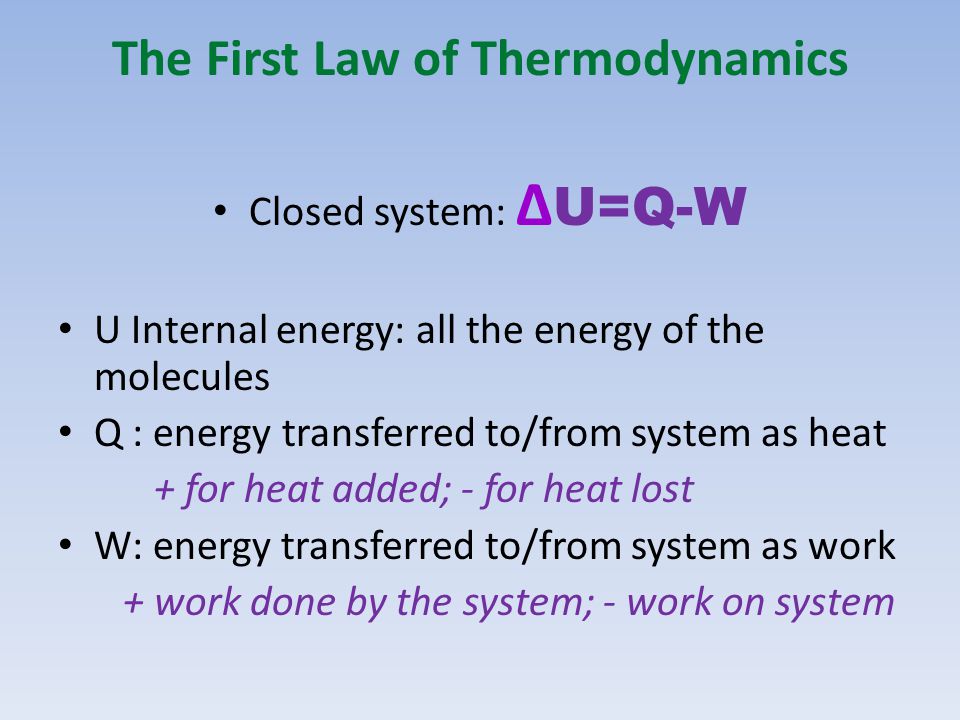
Thermodynamic Processes Illustrate how the 1 st law of thermodynamics is a statement of energy conservation Calculate heat, work, and the change in internal. - ppt download
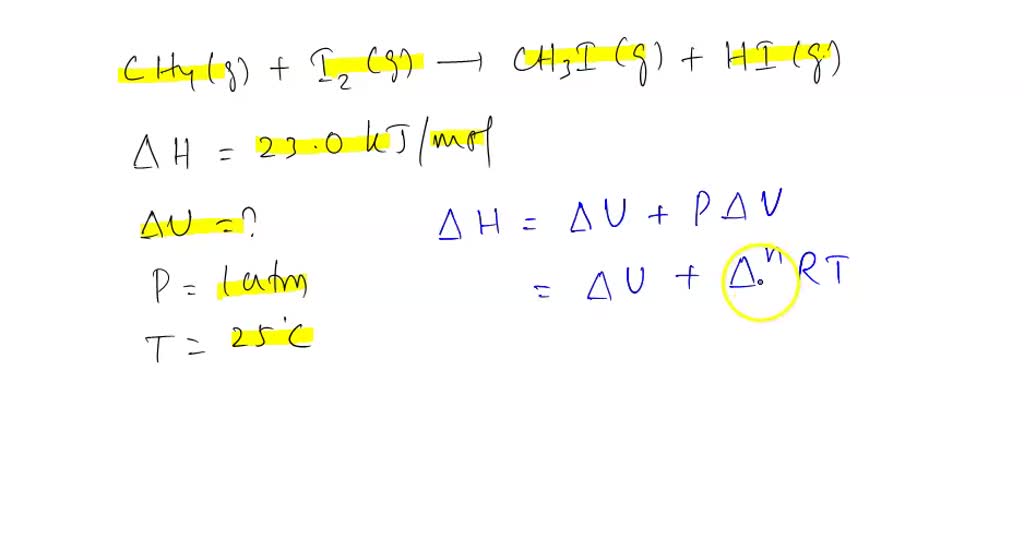
SOLVED: Calculate (delta)U for the following reaction at 1 atm and 25°C: CH4(g) + I2(g) —-> CH3I(g) + HI(g) (delta)H= +23.0 kJ/mol.

Calculate the value of Δ U for the following reaction: C2H4(g) + 3O2(g) → 2CO2(g) + 2H2O(l),Δ H = - 1410.0kJ
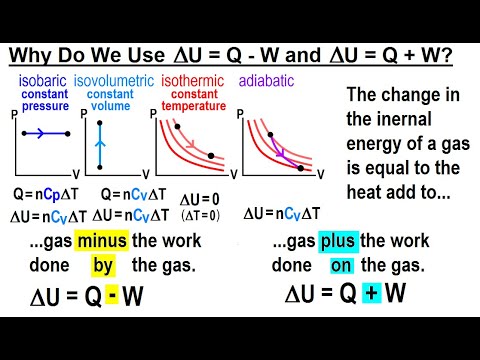
Physics: Viewer's Request: Thermodynamics #3: Why Do We Use (delta)U=Q-W and (delta)U=Q+W ? - YouTube
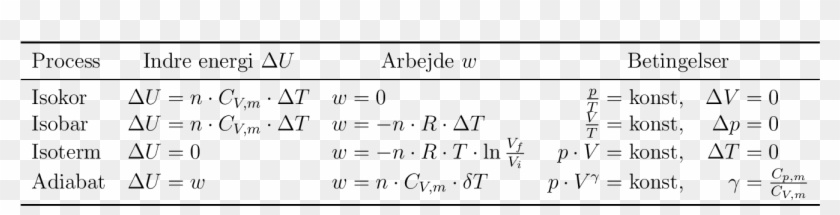
Compare the formula Cp - Cv = R for an ideal gas with the thermodynamics relation Delta U = Delta Q - P Delta V.
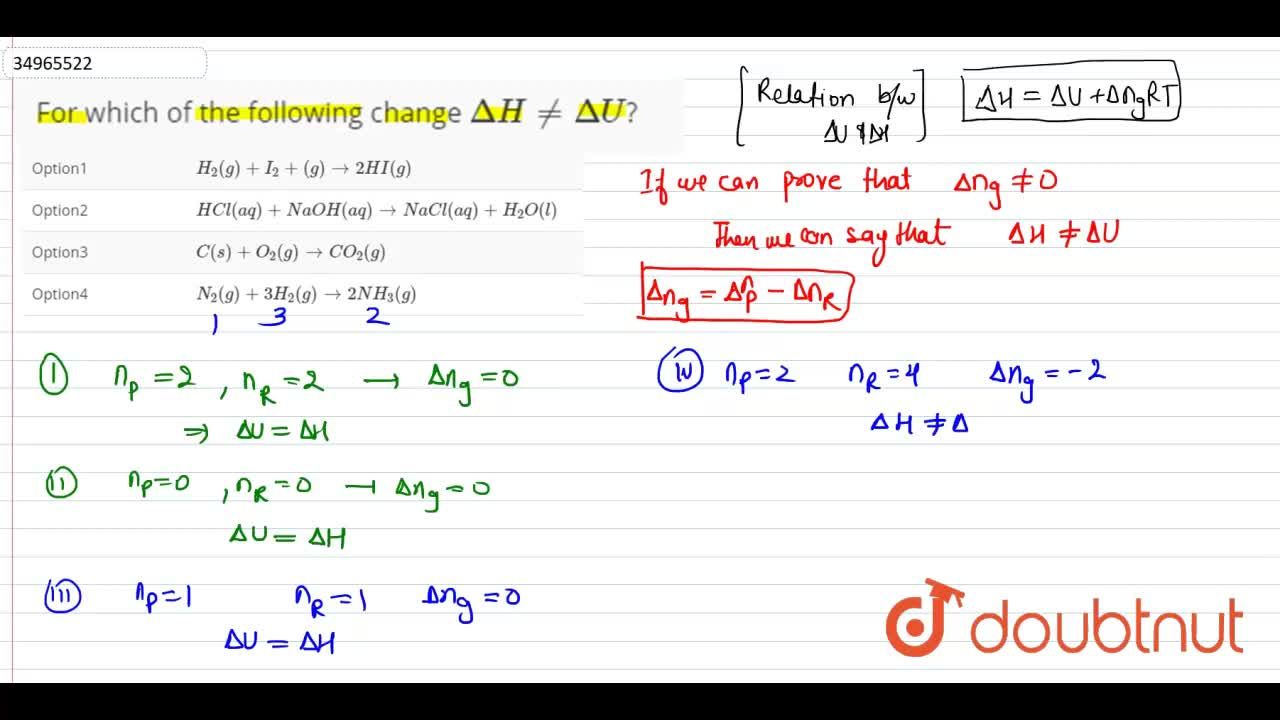
11. In which of the following delta H =delta U? (1) N2(g)+3H2(g)—> 2NH3(g) (2) c(s)+o2(g) >>CO2(g) (3)Pcl5(g) >>Pcl3(g)+cl2(g) (4)CaCO3(s) >>CaO(s)+CO2(g)